Controlling Oestrogen Without Pharmaceutical Drugs

In this blog, I’m going to review some of the most commonly investigated natural products in their roles as aromatase inhibitors. A number of men suffer from excess aromatisation of testosterone to oestrogen, resulting in an abnormal testosterone to oestradiol ratio. This can lead to negative symptoms such as anxiety, water retention, bloating, acne and gynaecomastia. We always advocate weight management and a healthy diet to reduce this risk, however it is sometimes still necessary to micro-dose an aromatase inhibitor until such time the ratio is normalised. All medications have potential side effects, the negative effects of aromatase inhibitors appear to mainly occur from inappropriate prescribing, something that can be avoided through careful dosing and regular follow up with your prescribing clinician. However, natural methods are always preferred, they are often inexpensive and can serve other important physiological purposes too. We will therefore explore what role nature can play in improving our oestrogen.
Why Do We Need Oestrogen?
Oestrogens serve important biological functions in the human body. This was already discussed in my first blog, The Importance of Oestrogen in TRT. To recap, oestrogen receptors exist throughout the body in different tissues and serve different roles. In the brain, there is a large number of oestrogen receptors in the pre-optic area, and the anterior hypothalamus contains a large amount of both aromatase and oestrogen receptors, both of which contribute to the regulation of libido 1. This also contributes to the regulation of positive mental wellbeing 2.
In the Testis, the Leydig, Sertoli, and Germ cells are abundant in aromatase and oestrogen, where oestrogens serve to modulate testosterone production and sperm production and quality 1. Oestrogen is therefore vital to maintaining fertility. Furthermore, the penile vasculature and the urothelium of the penis are lined with oestrogen receptors, where oestradiol modulates erectile function in the penis. This extends further to the cardiovascular system too, with oestrogen receptors within it that provide several protective effects on your cardiovascular system. This includes anti oxidative effects, increasing cell survival, an increase in vasodilation leading to lowered blood pressure, a decrease in reactive oxygen species production in the mitochondria reducing oxidation, an increase in the survival of mitochondria improving metabolism, in addition to decreased risk of fibrosis and atherosclerotic plaque build-up 3. Oestrogen is beneficial for bone and muscle tissue health and growth, too 4,5. Finally, oestrogens also have an important role in regulating glucose metabolism, insulin sensitivity and secretion, prevention of lipid accumulation and inflammation, and prevention of metabolic and liver diseases 6. Likewise, those who read my blog A Guide To Steroid Hormones & Immunity, will know that oestrogens also benefit immune function.
So, to summarise, oestrogens are essential to the following:
- Fertility (regulation of sperm quality, motility, & concentration), prevention of erectile dysfunction & positive regulation of libido
- Improved cardiovascular health
- Positive wellbeing
- Prevention of metabolic and liver disorders & better regulated blood sugar and lipids
- Prevention of excess fat gain
- Reduction in inflammation & improvement in immune system regulation
- Improved musculoskeletal health and growth
However, these positive effects consider a physiologically normal oestrogen level. If we are too low or too high in oestrogen, these effects diminish and issues arise. Therefore, we must control our oestrogen in accordance to the clinical symptoms experienced by the patient and their biochemical markers from blood work. This ensures optimal health and wellbeing.
A Recap – How we Get From Testosterone to Oestradiol & More
In men, the primary source of oestrogens is from the conversion of testosterone to oestradiol, catalysed by CYP19A1, which is also known as aromatase. This primarily occurs in adipose tissue (fat), though some oestrogen is produced in the testis too, with a small amount also coming from the adrenal glands.
This section may be complex, so bear with it if you’re willing to, otherwise feel free to skip this. Aromatase is a cytochrome P450 enzyme and it achieves this conversion via three successive hydroxylation’s of the 19-methyl group on androgens, then eliminating the methyl group as carboxylic acid (formate in this case), and aromatisation of the A-ring (hence, the name aromatase). Aromatase also converts Androstenedione (another steroid) to Estrone, a ‘weaker’ oestrogen which plays an important role in men in metabolic functions (high circulating estrone increases risk of diabetes) 7. This reaction looks like the following (click for full-size image):
For a condensed overview (click for full-size image):
Note that the A-ring is labelled for testosterone, where the aromatisation occurs.
Back to our main point, given that this is the primary source for oestradiol, if our serum oestradiol levels are too high, due to excess aromatisation, then we can intervene via pharmaceutical means to inhibit the action of aromatase. This is important, given that high oestradiol is associated with negative health outcomes which I also discussed in my first blog.
There are different modes of oestrogen metabolism, too. Estrone (E1), produced via conversion of Androstenedione by Aromatase, can convert to oestradiol (this is a reversible reaction via enzyme 17β-HSD2, so oestradiol, or E2, can convert back to E1 and vice versa), and various oestrogen metabolites which have a variety of functions in the body. This is shown below 8:
The biological functions of each metabolite go beyond the scope of this blog and so won’t be discussed, but do be aware that they all play important roles in your health. Increasing the number of metabolites produced and excreted (usually via urine) means a general reduction of overall serum E2, which tends to cause the most symptoms.
Another Recap – What Pharmaceutical Aromatase Inhibitors are Currently Available for Clinical Use?
As previously mentioned in the oestrogen blog, there are primarily two major classes of Aromatase Inhibitors. These include the following:
- Irreversible steroidal inhibitors (suicidal) such as Exemestane (Aromasin), which will permanently bind to the aromatase.
- Nonsteroidal inhibitors such as Anastrozole (Armidex), which reduces E2 via reversible competition (substrates, ie. testosterone and Armidex, will compete to bind with aromatase, but not kill the enzyme once the substrate is freed from aromatase).
When clinically necessary, The Men’s Health Clinic micro-doses Exemestane in order to normalise the testosterone to oestradiol ratio. This is because it has more of a fixed-dose response and there is no rebound effect on cessation.
What Can Raise Oestrogen?
The expression of aromatase (how much is produced and present) is greatest in adipose tissue. That gives reason to suggest that an increase in fat gain, be it visceral or subcutaneous, will result in an increase in oestrogens. This is precisely what we find in studies; your body composition can heavily affect your serum oestrogen and you will see me state this again later on, with additional studies 9. It’s therefore not surprising to hear that losing body fat can result in improved serum oestrogen levels.
Liver diseases are also known to contribute to elevated oestrogen as there is a high level of aromatase present in the liver too. Why may this be the case? Perhaps due to the potent antioxidant effects of oestrogen; if the liver is damaged, then an increase in oestrogen should help protect it 10,11. This may explain why liver cirrhosis is predominately a disease found in men and postmenopausal women. On the topic of the liver, alcohol can also increase oestrogen concentration, even when controlling for caloric intake 12–15. Diet and lack of exercise are also heavy contributors to elevated oestrogen, but I will talk about this more later.
Natural Products – What Do We Know?
Now that we’ve recapped on how we get from testosterone to oestradiol and how we can inhibit this process, let’s investigate what non-synthetic drugs may be of use in inhibiting aromatase and thus lowering oestradiol, or lowering it by other means. Inappropriate use of aromatase inhibitors can cause an increase in adverse cardiovascular events, alteration of lipid profiles, decreased bone mineral density, increased musculoskeletal disorders, and altered mental health, particularly cognitive ability 16–22. This is why aromatase inhibitors should ever only be used under the guidance of an experienced clinician, and only when absolutely necessary.
These AI side effects are rare and generally limited to high dose use, but results vary person to person, depending on various factors down to the individual and the choice of drug. Reduced side effects may well be seen with use of natural products, and some natural products may contain compounds that both inhibit aromatase and alleviate some side effects of oestrogen deprivation (eg. phytoestrogens can achieve this) 23. Additionally, natural AI products may also regulate aromatase via other pathways and receptors, such as the modulation of the liver protein liver receptor homologue-1 (LRH-1), which regulates aromatase in adipose tissue, the testis, and other tissue 24,25. Or, via receptor degradation.
Numerous natural product extracts have been tested and evaluated, mostly from edible plants and fungi, though also including spices, teas, coffees and cycads. However, the vast majority of this has been tested in vitro – in cell studies. Luckily, some preclinical and clinical studies have been performed.
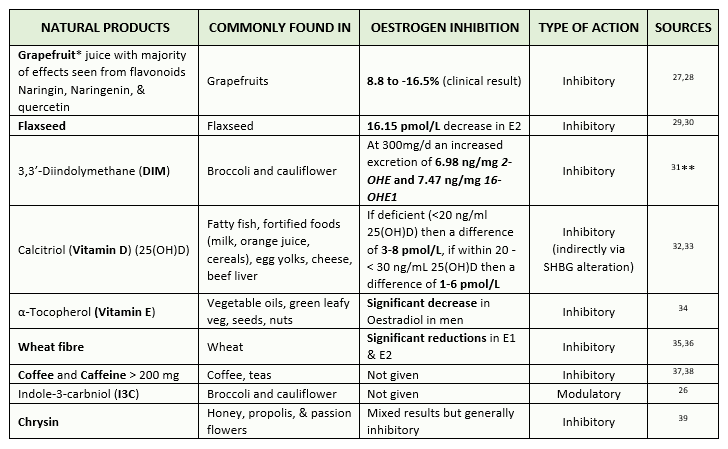
The number of natural products, extracts, and compounds is innumerable, so I’ve focused just on the top ten products, extracts or compounds of each category that has a high in vivo and/or in vitro activity/efficacy. I’ve then narrowed this down to find which compounds/extracts out of this list contain clinical studies with effectiveness, which led me to 9 natural products of most interest. Below I include a table of the said natural products, compounds and extracts that I’ve followed up for this blog, ranked from the most effective at reducing or modulating oestrogen at the top, down to not enough recorded clinical data present at the bottom. This list includes natural products that either inhibit oestradiol, or modulate its metabolism, ie. indole-3-carbinol (I3C) from cruciferous vegetables (e.g. cabbage and broccoli) increasing catechol oestrogen production, which generally has more favourable health effects than that of oestradiol 26.
Table 1 – Top 9 natural products, extracts & compounds that are known to reduce oestradiol both in vitro and in Humans within clinical studies. The level of in vitro inhibition was obtained from 23. Table is ranked from most effective to least/not enough recorded clinical data present (click for full-size image)
* Note that Grapefruit is a cytochrome P450 inhibitor which can alter medication absorption; do not take it if you’ve medicines that can interact with it.
** This study demonstrates that 300mg/d of DIM can increase the excretion of oestradiol metabolites, therefore it has a relatively strong antiestrogenic effect.
Bear in mind that this is not an exhaustive list of all the natural products we can use for the reduction of oestradiol, or for positive oestradiol metabolism. There are far more compounds of natural origin that are known to play a role in the control of oestrogen. For example, it’s known that Zinc, given to Zinc deficient rats, leads to reduced aromatisation of Testosterone, thus lowering oestrogen 40. It would be interesting to follow this up further in human clinical studies. However, there are few preclinical and clinical studies present and we can only garner so much information from rat or mouse studies; they don’t always translate to human models. Furthermore, most studies that investigate the roles of plant compounds on oestrogens are in peri or postmenopausal women. It’s therefore difficult to extrapolate and translate these results into men on TRT. But it does provide some insight into what we can expect to happen and is certainly better than using animal studies.
Please do not see this list as a recipe for an awful tasting cocktail of things you should take if you have high AI; consult your medical physician first and have blood tests performed. You should be aware of what side effects could present; just because something is natural doesn’t mean it won’t have side effects. Drug derivatives from plants are examples of this. Grapefruit, for example, is known to interact with other oral drugs. Likewise, you can overdose on Vitamin D or Caffeine. Studies demonstrating the effectiveness of broccoli in oestradiol inhibition required a hefty dose of 500g of broccoli. That’s quite a lot of broccoli and may cause stomach issues to those less tolerant. But, it’s clear that dietary modifications, correction of dietary deficiencies, or supplementation of certain compounds can favourably improve oestradiol concentrations and metabolism. On that note, diet does indeed have a profound impact on oestradiol concentrations, and I’ll discuss that next, alongside exercise.
Diet & Exercise Vs Oestrogen
Various micronutrients and different macronutrients, in addition to macronutrient composition, contribute to overall serum sex steroid hormone levels. This includes oestrogen. Dietary fibre is one contributor, with an increase fibre intake of up to 30g demonstrating significant reductions in oestrogen, versus those not consuming fibre in their diet.
In acute (short term and rapid) settings, fat seems to blunt the production of testosterone produced by testes 41, which is still of importance to men on HCG. Even in the long term, high fat intakes lower overall testosterone levels, but raises oestrogens and alter its metabolism negatively 42. With one rule to the exception though; an increased intake of omega-3 polyunsaturated fatty acids (as found in fatty fish/fish oil and flaxseed oil and other seeds) is associated with increased testicular volume, lower E2, greater free Testosterone and sperm quality, even when adjusting for other factors 43,44. Generally, we see a bell curve response with most epigenetic changes from fish oil alone, where it peaks at about 6g/day, or 1g EPA/DHA a day. Given the innumerable other benefits, it is advisable to increase your intake of fatty fish, or seeds containing EPA/DHA and aim for 1g a day (use supplementation if needed).
Less significant is your intake of carbohydrates and protein, however, an increased protein intake tends to lead to improved fat loss during caloric restriction and more muscle mass, in addition to better controlled blood glucose 45,46. Naturally, serum oestrogens levels decrease with a lowered body fat percentage too, as there has been less available fat to produce aromatase enzyme. Long term calorie restriction and exercise both also lead to increased serum total and free testosterone, as well as increased SHBG and lower E2, independent of bodyfat percentage 47.
Conclusions
After reading this, your take home on what to do should be clear. Eat more mono-unsaturated fatty acid rich foods such as salmon & tuna or flaxseed. Lower your caloric intake but maintain protein intake, exercise more often, and ensure that you get at least 25% of your caloric intake from fats. Eat more fibre and vegetables in general, particularly green and Cruciferous vegetables, in particular broccoli. Ensure that you get adequate intake of vegetables and fruit regardless, and drink plenty of water. Doing this, you will see improved oestradiol metabolism and lowered oestradiol. Will it be enough to control any symptoms of high oestradiol? That I can’t answer for you as responses will vary and natural products may therefore serve to replace an AI in some cases, but not all. Regardless, you’ll certainly be healthier by following these steps.
References
- Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl. 2016;18(3):435-440. doi:10.4103/1008-682X.173932
- Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010;2(1):153-166. doi:10.2147/ijwh.s6907
- Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33. doi:10.1186/s13293-017-0152-8
- Chidi-Ogbolu N, Baar K. Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 2019;10(JAN). doi:10.3389/fphys.2018.01834
- Väänänen HK, Härkönen PL. Estrogen and bone metabolism. Maturitas. 1996;23(SUPPL.). doi:10.1016/0378-5122(96)01015-8
- Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309-338. doi:10.1210/er.2012-1055
- Jasuja GK, Travison TG, Davda M, et al. Circulating estrone levels are associated prospectively with diabetes risk in men of the framingham heart study. Diabetes Care. 2013;36(9):2591-2596. doi:10.2337/dc12-2477
- Thomas MP, Potter BVL. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol. 2013;137:27-49. doi:10.1016/j.jsbmb.2012.12.014
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633-638. doi:10.1210/jcem-48-4-633
- Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23(1):63-69. doi:10.1034/j.1600-0676.2003.00811.x
- Longcope C, Pratt JH, Schneider S, Fineberg E. Estrogen and androgen dynamics in liver disease. J Endocrinol Investig Off J Ital Soc Endocrinol. 1984;7(6):629-634. doi:10.1007/BF03349497
- Hartman TJ, Sisti JS, Hankinson SE, Xu X, Eliassen AH, Ziegler R. Alcohol Consumption and Urinary Estrogens and Estrogen Metabolites in Premenopausal Women. Horm Cancer. 2016;7(1):65-74. doi:10.1007/s12672-015-0249-7
- Mahabir S, Pfeiffer R, Xu X, Baer DJ, Taylor PR. Effects of low-to-moderate alcohol supplementation on urinary estrogen metabolites in postmenopausal women in a controlled feeding study. Cancer Med. 2017;6(10):2419-2423. doi:10.1002/cam4.1153
- Purohit V. Can alcohol promote aromatization of androgens to estrogens? A review. Alcohol. 2000;22(3):123-127. doi:10.1016/S0741-8329(00)00124-5
- Rachdaoui N, Sarkar DK. Effects of Alcohol on the Endocrine System. Endocrinol Metab Clin North Am. 2013;42(3):593-615. doi:10.1016/j.ecl.2013.05.008
- Lønning PE. Bone safety of aromatase inhibitors versus tamoxifen. In: International Journal of Gynecological Cancer. Vol 16. ; 2006:518-520. doi:10.1111/j.1525-1438.2006.00685.x
- Gnant M. Management of bone loss induced by aromatase inhibitors. Cancer Invest. 2006;24(3):328-330. doi:10.1080/07357900600633759
- Chowdhury S, Pickering LM, Ellis PA. Adjuvant aromatase inhibitors and bone health. J Br Menopause Soc. 2006;12(3):97-103. doi:10.1258/136218006778234020
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-Year results of the anastrozole, tamoxifen, alone or in combination (ATAC) trial (18233230). J Bone Miner Res. 2006;21(8):1215-1223. doi:10.1359/jbmr.060508
- Markopoulos C, Polychronis A, Zobolas V, et al. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: Preliminary results of the TEAM Greek sub-study. Breast Cancer Res Treat. 2005;93(1):61-66. doi:10.1007/s10549-005-3783-0
- Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005;93:S23-S27. doi:10.1038/sj.bjc.6602692
- Rosenfeld CS, Shay DA, Vieira-Potter VJ. Cognitive effects of aromatase and possible role in memory disorders. Front Endocrinol (Lausanne). 2018;9(OCT). doi:10.3389/fendo.2018.00610
- Balunas M, Su B, Brueggemeier R, Kinghorn A. Natural Products as Aromatase Inhibitors. Anticancer Agents Med Chem. 2012;8(6):646-682. doi:10.2174/1871520610808060646
- Clyne CD, Kovacic A, Speed CJ, Zhou J, Pezzi V, Simpson ER. Regulation of aromatase expression by the nuclear receptor LRH-1 in adipose tissue. In: Molecular and Cellular Endocrinology. Vol 215. ; 2004:39-44. doi:10.1016/j.mce.2003.11.001
- Safi R, Kovacic A, Gaillard S, et al. Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor γ coactivator-1α on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005;65(24):11762-11770. doi:10.1158/0008-5472.CAN-05-2792
- Michnovicz JJ, Bradlow HL. Altered Estrogen Metabolism and Excretion in Humans Following Consumption of Indole-3-Carbinol. Nutr Cancer. 1991;16(1):59-66. doi:10.1080/01635589109514141
- Monroe KR, Stanczyk FZ, Besinque KH, Pike MC. The effect of grapefruit intake on endogenous serum estrogen levels in postmenopausal women. Nutr Cancer. 2013;65(5):644-652. doi:10.1080/01635581.2013.795982
- Schubert W, Eriksson U, Edgar B, Cullberg G, Hedner T. Flavonoids in grapefruit juice inhibit the in vitro hepatic metabolism of 17β-estradiol. Eur J Drug Metab Pharmacokinet. 1995;20(3):219-224. doi:10.1007/BF03189673
- Lopes CM, Dourado A, Oliveira R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed Res Int. 2017;2017:1-42. doi:10.1155/2017/7207983
- Sturgeon SR, Heersink JL, Volpe SL, et al. Effect of dietary flaxseed on serum levels of estrogens and androgens in postmenopausal women. Nutr Cancer. 2008;60(5):612-618. doi:10.1080/01635580801971864
- Rajoria S, Suriano R, Parmar PS, et al. 3,3′-diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: A pilot study. Thyroid. 2011;21(3):299-304. doi:10.1089/thy.2010.0245
- Zhao D, Ouyang P, de Boer IH, et al. Serum vitamin D and sex hormones levels in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas. 2017;96:95-102. doi:10.1016/j.maturitas.2016.11.017
- Krishnan A V., Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77(11):1107-1112. doi:10.1016/j.steroids.2012.06.005
- Mondul AM, Rohrmann S, Menke A, et al. Association of serum α-tocopherol with sex steroid hormones and interactions with smoking: Implications for prostate cancer risk. Cancer Causes Control. 2011;22(6):827-836. doi:10.1007/s10552-011-9753-4
- Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr. 1991;54(3):520-525. doi:10.1093/ajcn/54.3.520
- Monroe KR, Murphy SP, Henderson BE, et al. Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: The multiethnic cohort study. Nutr Cancer. 2007;58(2):127-135. doi:10.1080/01635580701327935
- Schliep KC, Schisterman EF, Mumford SL, et al. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am J Clin Nutr. 2012;95(2):488-497. doi:10.3945/ajcn.111.021287
- Wedick NM, Mantzoros CS, Ding EL, et al. The effects of caffeinated and decaffeinated coffee on sex hormone-binding globulin and endogenous sex hormone levels: A randomized controlled trial. Nutr J. 2012;11(1):86. doi:10.1186/1475-2891-11-86
- Hosseini Balam F, Ahmadi ZS, Ghorbani A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review. 2017. doi:10.1016/j.heliyon.2020.e03557
- OM A-S, Chung K-W. Dietary Zinc Deficiency Alters 5α-Reduction and Aromatization of Testosterone and Androgen and Estrogen Receptors in Rat Liver. J Nutr. 1996;126(4):842-848. doi:10.1093/jn/126.4.842
- Meikle AW, Stringham JD, Woodward MG, McMurry MP. Effects of a fat-containing meal on sex hormones in men. Metabolism. 1990;39(9):943-946. doi:10.1016/0026-0495(90)90305-V
- Pearce KL, Tremellen K. The effect of macronutrients on reproductive hormones in overweight and obese men: A pilot study. Nutrients. 2019;11(12). doi:10.3390/nu11123059
- Mínguez-Alarcón L, Chavarro JE, Mendiola J, et al. Fatty acid intake in relation to reproductive hormones and testicular volume among young healthy men. Asian J Androl. 2017;19(2):184-190. doi:10.4103/1008-682X.190323
- Jensen TK, Priskorn L, Holmboe SA, et al. Associations of Fish Oil Supplement Use With Testicular Function in Young Men. JAMA Netw open. 2020;3(1):e1919462. doi:10.1001/jamanetworkopen.2019.19462
- Campos-Nonato I, Hernandez L, Barquera S. Effect of a High-Protein Diet versus Standard-Protein Diet on Weight Loss and Biomarkers of Metabolic Syndrome: A Randomized Clinical Trial. Obes Facts. 2017;10(3):238-251. doi:10.1159/000471485
- Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, Araujo AB. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98(6):2442-2450. doi:10.1210/jc.2012-2582
- Cangemi R, Friedmann AJ, Holloszy JO, Fontana L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell. 2010;9(2):236-242. doi:10.1111/j.1474-9726.2010.00553.x