The Importance of Oestrogen in TRT
Micro-dosing Exemestane & Controlling Oestrogen – The Facts & the Why
Oestrogens are steroid hormones, and there are various Oestrogens (1). Estradiol (E2) is the predominant Oestrogen, and the Oestrogen that we’ll focus on. E2 is paramount to the maintenance of optimal health in both women and men. Though, as with nearly anything in life, balance is essential to ensure good health. If we have too much of something good, it can cause detrimental effects, either acutely, or in the long-run. This is also true for having too little of something that we need for good health.
I will first preface by mentioning the important and obvious fact that we are all genetically different. Even identical twins vary ever-so slightly and continue to vary as they grow older due to epigenetic changes brought on by environmental influences, such as your lifestyle (2). This genetic inter-individuality has given rise to personalised medicine; no one size fits all, especially when it comes to pharmaceutical intervention. We need to ensure we provide strategies that optimize drug therapy based upon each patient’s genetic determinants of drug efficacy and toxicity (3). As TMHC readers will know, it’s important to personalise their TRT according to their needs, bloodwork, and symptoms (both mental and physical).
So, what’d be our strategy when it comes to controlling Oestrogen in men? Why do we even care about Oestrogen in men? Let’s first dive into what Oestrogen does to us.
Oestrogen & Oestradiol – What is it?
I’ll first start with a very high-level biochemistry lesson on steroids.
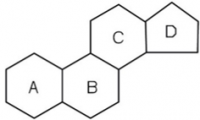
A steroid is simply a class of organic compounds which characteristically have a molecular structure containing four rings of carbon atoms. The very basic exoskeleton of a steroid would look like the following (click for full size image):
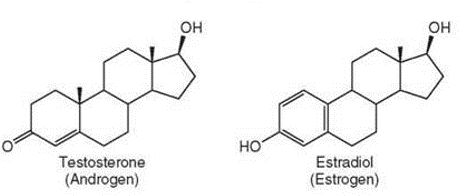
Each ring (A-C) has 6 points where the edges meet, which represents a carbon atoms. Ring D only has 5 carbon atoms. The addition of double bonds and hydroxyl groups (oxygen and hydrogen atoms bound together, or ‘OH’) would give rise to E2. E2 binds to its receptor, the Oestrogen receptor (ER) to mediate some of its Oestrogenic effects. It’s also similar in structure to testosterone, as we can see below (click for full size image):
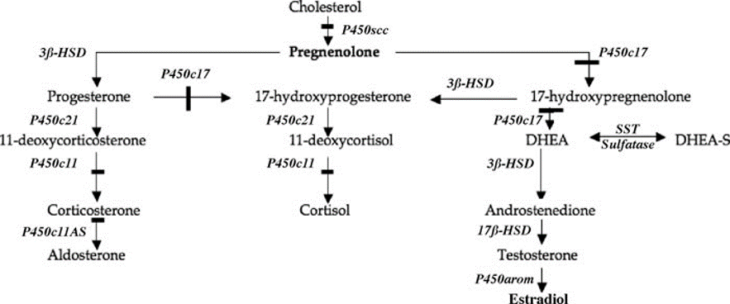
Looking at the two, it’s no surprise that testosterone is a precursor for E2. The conversion of Testosterone to E2 is catalysed by the aromatase (P450Aromatase) enzyme (a biological molecule that speeds up a chemical reaction). It’s by this reaction that the majority of E2 is made and it’s the steroidal biosynthetic pathway that ultimately gives rise to E2, among many other steroid hormones. This all starts with cholesterol and is well illustrated by the figure below (4) , where italic text represents an enzyme (click for full size image).
It’s clear from the figure that if we inhibit p450aromatase then we would reduce E2 production. Drugs called aromatase inhibitors (AIs) do exactly this.
What Does Oestradiol do for Men?
Within men, E2 plays a critical role with sexual function, modulating libido, erectile function, and spermatogenesis (5) It’s important for fertility. It’s also important for keeping excess pounds off, positive mood, muscle health, bone mineral density, joint health, and heart health.
The effects of E2 on libido alone starts with its direct effects in the brain. Oestrogen receptors (ERs) exist in the pre-optic area and the anterior hypothalamus regions of the brain, which also have high levels of aromatase and ER. These regions are known to be involved in increased sexual libido. Furthermore, if we give exogenous E2 to lower testosterone adult males, we observe an increase in libido (6). Interestingly, a case-study also existed where a male patient with aromatase deficiency and hypogonadism required both testosterone and E2 to increase libido; neither hormone alone could raise libido (6).
In their literature review, Schulster et al., 2016 identified that excess E2 increased the risk of erectile dysfunction (ED) irrespective of Testosterone levels (5). Likewise, low E2 can increase the risk of ED and animal models can give some insight into why this is the case; generally, the vasculature of the erectile tissue in the penis has a high ER density. E2 binds to the ER in this tissue and has an ‘anti-apoptotic’ effect on the endothelium of the penis (6). With excess E2, we can see a downregulation in ER density (7,8) and thus loss of this anti-apoptotic effect, and too little E2 will cause the same effect. Apoptosis is the process of programmed cell death. Therefore, adequate E2 is necessary to prevent the death of important penile vascular tissue and thus maintain good blood flow to the penis.
Aromatase exists in the Leydig cells of the testes, producing E2 which regulates spermatogenesis via several mechanisms. Excess E2 can lead to poor sperm concentration, motility, and morphology; administration of AIs has been shown to improve this. Likewise, too little E2 can decrease sperm quality (6). This is likely due to being linked to alterations in ER density and E2 concentration of the Leydig cells, thus highlighting the need to maintain appropriate E2 levels.
E2 is also known to regulate body composition (fat/muscle content) in men. Intervention studies have demonstrated that short-term E2 deprivation will contribute to increased fat gain (9). Why? E2 interacts with the hypothalamus of the brain to reduce food intake and reduces activity of lipoprotein lipase (LPL). LPL is a protein that’s key in promoting the uptake of fatty acids into fat cells, thus making one ‘fatter’. Though, with an excess of E2 we stop seeing this benefit. This is due to a complex interaction of another hormone called leptin, with E2 on the brain and the downregulation of ER density/sensitivity across the body (9,10). Fat cells have a high concentration of aromatase and thus E2 (in addition to leptin), so one method of E2 control would be to lose fat mass.
E2 is also known to lower the incidence of cardiovascular disease (CVD), a result of a combination of complex mechanisms (11). But, an excess of E2 conversely increases CVD risk. Again, this is primarily a result of decreased ER density/sensitivity and increased water retention (12). An increase in both intra and extracellular water is a side effect of excess E2 and increases in water retention have been shown to lead to a statistically significant increase in systolic blood pressure, which is indeed linked with CVD risk (13–15, 19). Though, the extent to how hazardous an excess E2 induced increase in water retention and CVD risk is something that has not been studied; it’s open to speculation. Nonetheless, we want to avoid increasing any risk factors for other diseases such as CVD.
But all this talk of too much or too little E2 is missing one key point: your Testosterone to Oestradiol ratio (T/E2). Your T/E2 is an incredibly important predictor or good health, provided we are within physiological normal levels of Testosterone and E2. Zheng et al., demonstrated that in their study, an imbalance of T/E2 lead to a statistically significant increased risk of CVD development, and using multiple linear regression (statistical method), they demonstrated a positive relationship between T/E2 and HDL-C (good cholesterol) (18). Other studies have also demonstrated the importance of the T/E2 ratio regarding other health complications. This includes libido and fertility, where a T/E2 of less than 10 has been associated with decreased semen quality and poorer libido (19). A longitudinal study (over 5 years) also demonstrated that a negative change in T/E2 ratio was inversely associated with a change in fat mass, irrespective of age; that’s to say that a lower T/E2 ratio increases the amount of fat you carry and changes where you carry it, causing it to be more centralised around the belly (20).
One major consideration that’s often overlooked is the role that estradiol plays in prostate cancer. It was commonly believed that in the past, androgen’s played the dominant role in increasing the risk of prostate cancer; though it has becoming clear from a knock-out study (knocking out genes in mice) by Ellem and Risbridger, 2010 (27), that Testosterone only serves to cause prostatic hypertrophy and hyperplasia and NOT prostate cancer, whereas Estradiol being too high indeed did. Of course, you’re not a mouse. However, these results appear to be consistent among male populations, and it is increasingly more obvious when looking at the elderly male populations who’s testis decrease the level of Testosterone production, without a change in Estradiol. What appears to be the main culprit is an excessively high E2 with a poor T/E2 ratio (26). Of particular worthy note is the different ERs; ER-alpha encourages cell survival and proliferation, whereas the ER-beta receptor encourages cell death. Thus, ER-α binding of E2 is morel likely to cause prostate cancer due to rapid growth of prostate cells (proliferation) and survival of cancerous cells. The process of apoptosis is still present during hyperplasia induced by androgen’s, thus ensuring that cancerous cells are less likely to survive and more likely to be killed by your immune system. In-fact, ER-β agonists are being investigated for potential prostate cancer treatment due to the fact that they promote cell-death among prostate cells (26).
From the few explanations and examples of E2s positive effects, it’s clear that we need it to be healthy. However, it’s not without its side effects, which include gynecomastia and increased water retention, as mentioned (16,17). Clearly, moderation is key! We need E2 to be healthy, but we need to keep it at a level that provides the best health outcome for a patient. We also need to have an appropriate T/E2 level, which will vary patient to patient and thus we need to first control their E2 to assess what works best for them. We can control E2 via AI’s.
Aromatase Inhibitors, Classes & Micro-dosing
As mentioned, aromatase inhibitors (AIs) will lower E2. The mechanism by which they inhibit Aromatase depends on the type, and there are two major classes:
- Irreversible steroidal inhibitors (suicidal), such as Exemestane (Aromasin), which will bind permanently to the Aromatase
- Nonsteroidal inhibitors, such as anastrozole (Armidex) which reduces E2 via reversible competition (substrates, i.e. testosterone and Armidex, will compete to bind with Aromatase, but not kill the enzyme once the substrate is freed from Aromatase).
Too much Exemestane can cause a ‘crash’ of E2 levels that won’t increase until more aromatase is created. But we should always take a cautious approach and start with smaller doses to assess patient tolerance.
So… which is better? The answer is it depends on the situation. Both AIs are clinically safe, as demonstrated in clinical studies (14–16). For example, Zhao et al., demonstrated that in post-menopausal women being treated for breast cancer, no third-generation AIs (anastrozole, letrozole, and exemestane) increased the risk of thromboembolisms and CVD risk. Though the majority of data is within female populations, some studies have been performed in men and show carry-over. For example, Tan et al., 2014 performed a litreature review, searching for studies using AIs in adult men to assess clinical safety. They identified that positive effects were reported and the adverse effects on bone health that are seen within females using AIs aren’t seen in males (24). Although this is only true in cases with a warranted need to use AI’s, i.e. symptomatic of high E2 with a poor T/E2 ratio. This is well demonstrated when using letrozole in men with a normal T/E2 ratio (>10), but where participants didn’t have excess E2, where adverse effects were reported after 4 months, including a loss of libido, headaches, fatigue, weakness, loss of hair, and dry mouths (25). This ratio was calculated using Testosterone in ng/dl and oestradiol in pg/ml. Nonetheless, provided we are treating symptomatic males with poor T/E2 ratios and high levels of E2 that need AI’s, AI’s are clinically safe to use.
Due to the difference in how Exemestane and Anastrozole interact with the body, exemestane is far easier to control and titrate than armidex and it ensures that we don’t see a rebound in E2 the moment we titrate dosages downwards. We also won’t see a rebound in E2 if a patient forgets to take their AI dose, which could otherwise cause detrimental effects such as ED. Furthermore, the half-life of Exemestane is 27 hours versus Anastrozole’s 41-48 hours, meaning that for Exemestane to produce a baseline level, we’d require daily dosing; Exemestane can be titrated down to a more suitable dose far sooner.
Therefore, at TMHC, we prefer to use Exemestane with our patients as we can more precisely titrate it and thus better control E2 without fear of E2 rebound. We take smaller, more frequent doses (microdose), as opposed to taking large bolus doses, due to this reduced half life and ease of titration. We can therefore build up a baseline value and assess tolerance along the way, ensuring we have the best AI dose (based upon the patients E2 and symptoms).
We’re currently working on an algorithm that will ensure optimized E2 levels in every patient, dependent upon their symptoms. Microdosing and use of Exemestane is essential to this algorithm and helps optimise your health and well-being, thus ensuring you get the gold standard treatment you deserve.
References
- Coelingh Bennink HJT. Are all estrogens the same? In: Maturitas. Vol 47. ; 2004:269-275. doi:10.1016/j.maturitas.2003.11.009
- Wong CCY, Caspi A, Williams B, et al. A longitudinal study of epigenetic variation in twins. Epigenetics. 2010;5(6):516-526. doi:10.4161/epi.5.6.12226
- Evans WE, Johnson JA. Pharmacogenomics : The Inherited Basis for Interindividual Differences in Drug Response. Annu Rev Genomics Hum Genet. 2001;2(1):9-39. doi:10.1146/annurev.genom.2.1.9
- Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: Mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179(January 2018):19-40. doi:10.1111/j.1749-6632.2009.04980.x
- Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl. 2016;18(3):435-440. doi:10.4103/1008-682X.173932
- Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: Clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185(1):17-23. doi:10.1016/j.juro.2010.08.094
- Lee YJ, Gorski J. Estrogen receptor down-regulation is regulated noncooperatively by estrogen at the transcription level. Mol Cell Endocrinol. 1998;137(1):85-92. doi:10.1016/s0303-7207(97)00235-9
- Borrás M, Hardy L, Lempereur F, et al. Estradiol-induced Down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol. 1994;48(4):325-336. doi:10.1016/0960-0760(94)90072-8
- Rubinow KB. Estrogens and body weight regulation in men. In: Advances in Experimental Medicine and Biology. Vol 1043. Springer New York LLC; 2017:285-313. doi:10.1007/978-3-319-70178-3_14
- Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol – Endocrinol Metab. 2008;294(5). doi:10.1152/ajpendo.00733.2007
- Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33. doi:10.1186/s13293-017-0152-8
- Zheng HY, Li Y, Dai W, Wei CD, Sun KS, Tong YQ. Imbalance of testosterone/estradiol promotes male CHD development. In: Bio-Medical Materials and Engineering. Vol 22. ; 2012:179-185. doi:10.3233/BME-2012-0705
- Hür E, Özişik M, Ural C, et al. Hypervolemia for hypertension pathophysiology: A population-based study. Biomed Res Int. 2014;2014. doi:10.1155/2014/895401
- Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96(3):1011-1018. doi:10.1152/japplphysiol.01032.2003
- Wu CY, Hu HY, Chou YJ, Huang N, Chou YC, Li CP. High blood pressure and all-cause and cardiovascular disease mortalities in community-dwelling older adults. Med (United States). 2015;94(47):e2160. doi:10.1097/MD.0000000000002160
- Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B. Gynecomastia: Clinical evaluation and management. Indian J Endocrinol Metab. 2014;18(2):150-158. doi:10.4103/2230-8210.129104
- Stachenfeld NS. Hormonal changes during menopause and the impact on fluid regulation. Reprod Sci. 2014;21(5):555-561. doi:10.1177/1933719113518992
- Gong Y, Xiao H, Li C, et al. Elevated T/E 2 Ratio Is Associated with an Increased Risk of Cerebrovascular Disease in Elderly Men. PLoS One. 2013;8(4). doi:10.1371/journal.pone.0061598
- Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril. 2012;98(6):1359-1362. doi:10.1016/j.fertnstert.2012.10.023
- Wu A, Shi Z, Martin S, Vincent A, Heilbronn L, Wittert G. Age-related changes in estradiol and longitudinal associations with fat mass in men. Bjornstad P, ed. PLoS One. 2018;13(8):e0201912. doi:10.1371/journal.pone.0201912
- Zhao X, Liu L, Li K, Li W, Zhao L, Zou H. Comparative study on individual aromatase inhibitors on cardiovascular safety profile: A network meta-analysis. Onco Targets Ther. 2015;8:2721-2730. doi:10.2147/OTT.S88179
- Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005;93:S23-S27. doi:10.1038/sj.bjc.6602692
- Markopoulos C, Polychronis A, Zobolas V, et al. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: Preliminary results of the TEAM Greek sub-study. Breast Cancer Res Treat. 2005;93(1):61-66. doi:10.1007/s10549-005-3783-0
- Tan RBW, Guay AT, Hellstrom WJG. Clinical use of aromatase inhibitors in adult males. Sex Med Rev. 2014;2(2):79-90. doi:10.1002/smrj.23
- Chan JKY, Shuling L, Lau M, et al. Aromatase inhibitor Letrozole Male infertility Oligozoospermia Sperm concentration Testosterone-oestradiol ratio Do men with normal testosterone-oestradiol ratios benefit from letrozole for the treatment of male infertility? Reprod Biomed Online. 2018;38:39-45. doi:10.1016/j.rbmo.2018.09.016
- Di Zazzo E, Galasso G, Giovannelli P, Di Donato M, Castoria G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front Oncol. 2018;8:2. Published 2018 Jan 18. doi:10.3389/fonc.2018.00002
- Stuart J. Ellem, Gail P. Risbridger. Aromatase and regulating the estrogen: Androgen ratio in the prostate gland. Journal of Steroid Biochemistry and Molecular Biology, 118, 4-5, 2. Published 2010.