Stress & Testosterone
In this piece, I’ll be talking about the relationships between testosterone and stress. We’ll explore questions such as:
- Does stress lower testosterone?
- What is stress?
- Is stress catabolic – Are stress hormones killing my gains?!
- How do stress and testosterone relate?
What Really is Stress?
Everybody has experienced some form of stress in their life, especially now during this stressful time. The word ‘stress’ is often understood by many, without understanding what it is and what the innate biological response to it is. There are various views and language differences in using the terms stress which often leads to confusion in scientific and general communities.
A good biomedical definition would be that from Tsigos et al., 2016, which describes states that stress is a state of threatened homeostasis which is caused by intrinsic of extrinsic adverse forces (stressors). It’s counteracted by an intricate repertoire of physiologic and behavioural responses which aim to maintain the bodies optimal equilibrium (Tsigos et al., 2016).
To reiterate that sentence in simple English, stress is a state that threatens our bodies natural physiological balance (deviating from your normal healthy state). It’s caused by stressors, i.e. work, traumatic events, exercise, and social events. Stress is counteracted by biological changes (i.e. hormonal changes) and behavioural changes (i.e. anxiety, aggression, sadness, anger, or other emotions) to ultimately return to the baseline healthy and optimal state. Though, as we know, returning to a normal state can take quite some time.
The stress response depends upon the highly interconnected neuroendocrine stress system which is comprised of cellular and molecular infrastructure. Key components of the system include the hypothalamic-pituitary-adrenal (HPA) axis, and autonomic nervous system (ANS), which interact with the central nervous system (CNS) and peripheral tissues/organs to produce a successful adaptive response against the stressor. Dysregulation of this system (both hyper and hypo-activation) can lead to a marked disruption in body homeostasis leading to clinical complications.
Removing from the purely biological definition, I must stress (no pun intended) that there’s arguably differing levels of stress, including “good stress”, such as rising to a challenge, taking the risk, and feeling rewarded by a positive outcome. There’s “tolerable stress”, situations where bad things happen but you are able to cope through it with the support of family, friends, and other individuals, and “toxic stress” where such support is not present. Toxic stress can quickly turn into chronic, long term stress, which has adverse effects on your behaviour and physiology (McEwen, 2017).
The Stress Process
The stress system is very complex, so I’ll cut to the main neuroendocrinal point for this blog, which is best described with an example.
You’re driving down the motorway at the speed limit, the car in front of you suddenly breaks to a full stop. You slam on your breaks and come to a full halt, just before striking the back of the car in front of you. Your heart rate and blood pressure have skyrocketed, you feel shaky, maybe in disbelief, with thoughts racing through your mind. Cortisol is one of many stress hormones responsible for this physiological change.
What’s happened here to cause these physiological responses? The amygdala in your brain, responsible for processing fear, arousal, and emotional stimuli to determine the best response, sends a stress signal to your hypothalamus.
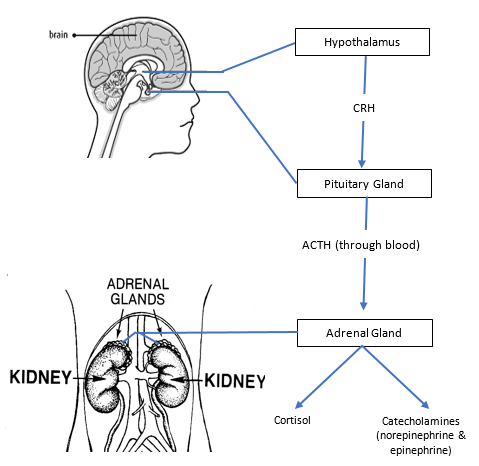
Your hypothalamus (more so the paraventricular nucleus in the hypothalamus) will respond by secreting corticotrophin-releasing factor (CRF), which signals the pituitary gland to secrete adrenocorticotropic hormone (ACTH). ACTH will bind to receptors in the cortex of the adrenal gland of the kidneys and lead to an increased production of cortisol. Catecholamines are also released, including epinephrine (adrenaline), norepinephrine, and aldosterone – this is achieved via stimulation of the adrenal medulla in the kidneys by the splanchnic nerves in the sympathetic nervous system (SNS) (Thau and Sharma, 2019). Glucagon is also released by the alpha cells of the pancreas and acts to increase blood glucose and free fatty acids for more easily available chemical energy. Cortisol release is shown in the picture below, sourced from university of Hampshire (click for full-size image):
This acts to increase cortisol and catecholamines in the blood stream. The adrenaline causes tachycardia (high heart rate), hypertension, diaphoresis (sweating), an increase in respiratory rate (breathing), and an increase in blood glucose. The cortisol helps the body stay on high alert; once the threat passes the parasympathetic nervous system will reduce the SNS response to return to optimal hormone balance.
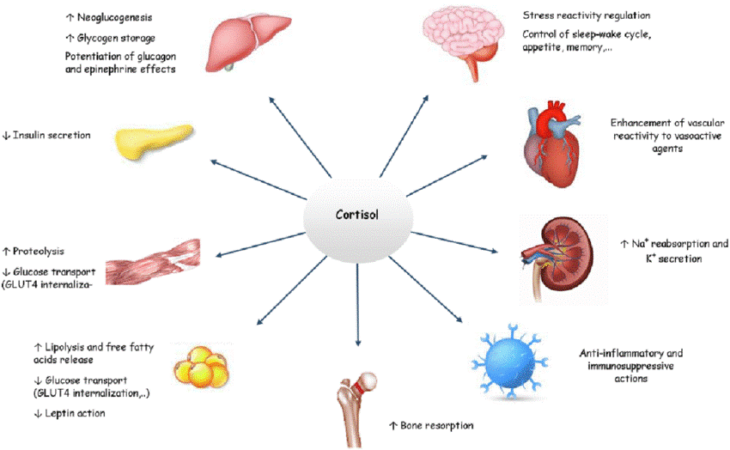
What does cortisol generally do? I think this is best described in a diagram, as made by (Oprea et al., 2019) (click for full-size image):
If you’ve read the previous blog, you’ll know that cortisol acts to regulate the metabolism of glucose, lipids, and proteins, in addition to controlling the immune response. It also alters electrolyte balance (increase sodium retention and excrete more potassium leading to water retention), reduces bone formation, helps (alongside adrenaline) create short-term memories based on emotional events, and alters appetite and mood.
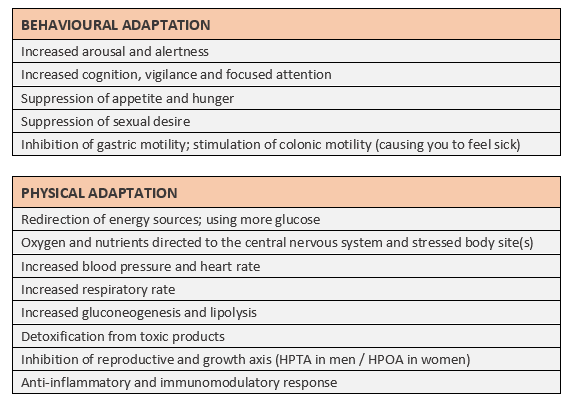
The table below summarises a list of behavioural and physical changes during stress (Adapted from Tsigos et al., 2016) (click for full-size image).
All these things help protect you from the stressor; this is the fight or flight system response. Generally, such a response would be short lived. But, as we’re aware – our stressors can continue.
In our example, nobody would’ve been hurt, but both cars are damaged. Your insurer puts you at fault and you need to pay an excess to fix your car, but you don’t have the money to do it. You need your car for work. I think you know what happens with the rest of the story, the bottom line is that your acute stressor has turned into a chronic stressor as the days go by. You’re constantly worried. Sometimes, the stressor passes but the response remains, too. This can occur with changes in neural circuitry and often requires pharmacological and/or therapeutic intervention.
In such a situation, we are presented with chronic stress. Cortisol is elevated, insulin resistance occurs, you may be heading towards diabetes, weight gain, we get ill more often, your eating patterns change, and mental health issues may arise (Yan et al., 2016; Li et al., 2013; Marcovecchio and Chiarelli, 2012). Chronic stress can also lead to the inhibition of skeletal muscle and bone growth. Things can be more sinister yet, as the suppression of the immune system leads to decreased cytotoxic T lymphocyte and natural killer cell activity, leading to growth of malignant cells, genetic instability, and tumour expansion (Reiche et al., 2004).
But, don’t stress over stress. We need to learn to cope with our stress, be it via support networks from others, or through, meditation, a hobby, or some kind of activity. Stress is a vital physiological response that helps us survive, and sometimes rise to challenges and see through to the end of them. Exercise is one form of a stressor, but it leads to numerable benefits, including an increased buffer against stress.
Anabolic vs Catabolic Hormones & Stress
I’ve added this section to this blog as I think it’s quite useful to also understand the wider implications that each hormone involved in the HPTA, with stress, have on anabolism and catabolism. I’ll focus primarily on body composition and metabolism.
Anabolism refers to the synthesis of complex organic molecules in living organisms, from simpler molecules, i.e. creation of glycogen from glucose in the liver or muscles; the product of glycogen synthesis (glycogenesis). Or, the creation of actin and myosin protein filaments in muscles from amino acids, the by-product of protein synthesis.
Catabolism refers to the breakdown of complex organic molecules in living organisms from complex ones to simpler ones i.e. the conversion of glycogen to glucose in the liver, a process called glycolysis.
STRESS HORMONES
Generally, stress hormones act to cause a net catabolic state where there’s a downregulation of synthesis. This acts to provide more readily available chemical substrate (glucose and fatty acids) for energy to fight or run away from the stressor. Protein catabolism can occur to meet blood glucose demands via gluconeogenesis.
Glucocorticoids
Glucocorticoids,such as cortisol, are largely catabolic in nature. In many tissues, including muscle, skin, lymphoid, connective, and adipose (fat) tissue, they cause a decrease in synthesis and increased degradation of protein and RNA. They also inhibit glucose and amino acid uptake and enhance lipolysis. This is largely catabolic, and necessary in times of stress, including acute exercise induced stress. Contrastingly, in the liver we see some anabolic effects, including an increase in protein and glycogen content (Baxter and Forsham, 1972).
Growth Hormone
Growth hormone can be viewed as the primary anabolic hormone during stress and fasting, via stimulation of IGF-1. In children and those going through puberty, GH is particularly important for the growth of lean body mass. In adults, it encourages increase water retention, but is nonetheless anabolic in connective tissues, certain other tissues, and bone, encouraging synthesis. It can encourage metastatic growth; ergo an increased risk of tumour development. In adipose tissue it is catabolic, encouraging lipolysis (fat break down), likewise it encourages glycolysis which causes an increase in blood glucose. Hence why chronic exposure of high levels of GH can lead to development of type 2 diabetes. Insulin resistance is a significant defence against hypoglycaemia, but when chronic, it’s rendered more harmful due to the ease of food availability in our modern society (Moøller and Joørgensen, 2009).
Epinephrine
Epinephrine is catabolic in adipose tissue and encourages glycolysis. Though, it has an anti-catabolic effect on protein metabolism (it prevents catabolism of protein but does not encourage protein synthesis). This may largely be mediated by an increase in insulin resistance, which spares muscle protein but can be problematic in a longer time period as it can lead to the development of type 2 diabetes mellitus (Umpleby and Russell-Jones, 1996).
Glucagon
Glucagon is catabolic in the sense that it encourages lipolysis and glycolysis. It works in a negative feedback loop with insulin – when blood glucose is lowered, glucagon is released by the alpha cells of the pancreas to raise blood glucose and fatty acids in the blood stream. It’s elevated during a period of stress.
SEX-STEROID HORMONES
The primary sex-steroid hormones have a net anabolic effect, as one would expect.
Testosterone
Testosterone stimulates skeletal muscle protein synthesis and inhibits protein degradation, they also encourage bone formation and connective tissue growth (Astrid M. Horstman, E. Lichar Dillon, Randall J. Urban, 2012; Mohamad et al., 2016). Testosterone acts to increase lipolysis via upregulation of the adrenoreceptor (receptor for catecholamines like adrenaline) (Lee et al., 2013).
Oestrogens
Oestrogens appear to have a beneficial effect of muscle strength and are likely anabolic in the muscle by encouraging a pro-anabolic muscle environment; oestrogens upregulate mRNA which positively regulate skeletal muscle growth, and downregulate mRNA that negatively regulates skeletal muscle growth (Tiidus, 2011). Oestrogens also encourage anabolism in connective tissue via collagen synthesis, in addition to bone formation (Chidi-Ogbolu and Baar, 2019). It also has both pro and anti-lipolytic roles, which is beyond the scope of this blog (Rubinow, 2017).
Sex steroid hormones have a complex relationship with glucocorticoids, catecholamines, the GH-IGF-1 axes, and insulin and glucagon.
The Main Topic – How Will Stress Affect Testosterone?
This question is multifactored. It depends upon what the cause of the stress response was, and whether it’s chronic or acute. Acute stress tends to lower Testosterone, but only temporarily (Tsigos et al., 2016).
Take for instance, excessive exercise. The term ‘over-training’ comes to mind, something misused by people quite often. Excessive exercise can be problematic by having excessive overloading in training stress with a lack of adequate recovery. Often, we may see a reduction in Testosterone to the lower end of ‘clinically normal’, particularly true withexcessive endurance training (Hackney and Aggon, 2018). We can also note a decrease in free Testosterone in those over trained with resistance training (i.e. American football players during an intensive pre-season training regimen) In extreme combat sports, we generally will see small increase in Testosterone, even with the continued fight or flight response, most likely a result of stress buffering. However, overtraining (particularly among the elite) may well again lead to a decrease in Testosterone, concurrent with an increase in Testosterone. (Anderson et al., 2016; Slimani et al., 2018).
What about those with post-traumatic stress disorders (PTSD)? This is where things become difficult to elucidate. Some studies suggest a decrease (Mulchahey et al., 2001), whereas some suggest that in combat related PTSD, there’s no differences compared to normal populations (Spivak et al., 2003), argued to be due to adaptations of the HPTA against stress in those with combat related PTSD. This is a difficult question to answer as lower levels of testosterone prior to the onset of PTSD often correlate with a higher risk for PTSD (Michopoulos et al., 2015). So, it depends upon the type of PTSD being dealt with; among combat related PTSD from men in the military, we see no differences in Testosterone and free testosterone. But sexual dysfunction is a common complaint, and major depression is comorbid with PTSD. Furthermore, we do see disordered eating behaviours among those with PTSD, which can lead to malnutrition and excessive weight gain or loss; both can very well lead to lowered testosterone and free testosterone (Mitchell and B. Porter, E. J. Boyko, 2016; Pasquali, 2006). It’s likely that the effect of PTSD varies among population groups, where military soldiers are trained to become used to high stress situations and adapt to them, thus we do not see the expected endocrinal disruption.
Generally, chronic stressors will lead to a reduction in Testosterone due to the effects of the HPA in response to stress. Cortisol and testosterone have an inverse relationship, and testosterone tends to limit the stress response (Pasquali, 2012; Rubinow et al., 2005). Stress can very well lower Testosterone, and it may be a cause of low Testosterone in many cases; this is something to consider before jumping to gun on being treated with TRT, if you are perhaps over trained or stressed out, as it may be something you are able to reverse and treat yourself.
Conclusion
Stress is essential. It helps us adapt to stressors. But, when we are left unable to cope with stress and it becomes a chronic problem, we need to seek help as it becomes harmful to our health. The development of non-communicable diseases such as overweightness and type 2 diabetes mellitus can help contribute to poorer health and reduced Testosterone. Testosterone can be directly reduced by stress hormones.
Dealing with stress can be essential. The time to get help when stressed out depends how stressed you are, whether you have the support in place to deal with it, the type of stressor, and how you’re responding with it. It’s imperative you make sure you have other people by your side that you can talk to if you ever need to and take the opportunity to seek medical professional help if needed as stress can cause mental health complications. Don’t be silent, don’t hesitate to speak up and get help. Be it at the NHS, or from family.
References
- Anderson,T. et al. (2016) CHANGES IN RESTING SALIVARY TESTOSTERONE, CORTISOL AND INTERLEUKIN-6 AS BIOMARKERS OF OVERTRAINING. J. Sport Heal. Sci., 2, 2–7.
- Astrid M. Horstman, E. Lichar Dillon, Randall J. Urban, and M.S.-M. (2012) The Role of Androgens and Estrogens on Healthy Aging and Longevity. J Gerontol A Biol Sci Med Sci, 1140–1152.
- Baxter,J.D. and Forsham,P.H. (1972) Tissue effects of glucocorticoids. J. Med., 53, 573–589.
- Chidi-Ogbolu,N. and Baar,K. (2019) Effect of estrogen on musculoskeletal performance and injury risk. Physiol., 10.
- Hackney,A.C. and Aggon,E. (2018) Chronic Low Testosterone Levels in Endurance Trained Men: The Exercise- Hypogonadal Male Condition. Biochem. Physiol., 1.
- Lee,H.-K. et al. (2013) The Role of Androgen in the Adipose Tissue of Males. World J. Mens. Health, 31, 136.
- Li,L. et al. (2013) Acute psychological stress results in the rapid development of insulin resistance. Endocrinol., 217, 175–184.
- Marcovecchio,M.L. and Chiarelli,F. (2012) The effects of acute and chronic stress on diabetes control. In, Science Signaling.
- McEwen,B.S. (2017) Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress, 1, 247054701769232.
- Michopoulos,V. et al. (2015) Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Psychiatry, 78, 344–353.
- Mitchell,K.S. and B. Porter, E. J. Boyko, and A.E.F. (2016) Longitudinal Associations Among Posttraumatic Stress Disorder, Disordered Eating, and Weight Gain in Military Men and Women. J. Epidemiol., 33–47.
- Mohamad,N.V. et al. (2016) A concise review of testosterone and bone health. Interv. Aging, 11, 1317–1324.
- Moøller,N. and Joørgensen,J.O.L. (2009) Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Rev., 30, 152–177.
- Mulchahey,J.J. et al. (2001) Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology, 26, 273–285.
- Oprea,A. et al. (2019) Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Adv. Endocrinol. Metab., 10, 204201881882129.
- Pasquali,R. (2006) Obesity and androgens: facts and perspectives. Steril., 85, 1319–1340.
- Pasquali,R. (2012) The hypothalamic-pituitary-adrenal axis and sex hormones in chronic stress and obesity: Pathophysiological and clinical aspects. N. Y. Acad. Sci., 1264, 20–35.
- Reiche,E.M.V. et al. (2004) Stress, depression, the immune system, and cancer. Lancet Oncol., 5, 617–625.
- Rubinow,D.R. et al. (2005) Testosterone Suppression of CRH-Stimulated Cortisol in Men. Neuropsychopharmacology, 30, 1906–1912.
- Rubinow,K.B. (2017) Estrogens and body weight regulation in men. In, Advances in Experimental Medicine and Biology. Springer New York LLC, pp. 285–313.
- Slimani,M. et al. (2018) Hormonal responses to striking combat sports competition: A systematic review and meta-analysis. Sport, 35, 121–136.
- Spivak,B. et al. (2003) Plasma testosterone levels in patients with combat-related posttraumatic stress disorder. Neuropsychobiology, 47, 57–60.
- Thau,L. and Sharma,S. (2019) Physiology, Cortisol StatPearls Publishing.
- Tiidus,P.M. (2011) Benefits of Estrogen Replacement for Skeletal Muscle Mass and Function in Post-Menopausal Females: Evidence from Human and Animal Studies. Eurasian J. Med., 43, 109–114.
- Tsigos,C. et al. (2016) Stress, Endocrine Physiology and Pathophysiology MDText.com, Inc.
- Umpleby,A.M. and Russell-Jones,D.L. (1996) The hormonal control of protein metabolism. Clin. Endocrinol. Metab., 10, 551–570.
- Yan,Y.X. et al. (2016) Investigation of the relationship between chronic stress and insulin resistance in a Chinese population. Epidemiol., 26, 355–360.