TRT & Fertility – Microdosing Testosterone & HCG
Commencing Testosterone Replacement Therapy (TRT) is not a small decision for one to take. There are a multitude of considerations to be made. The first one being, is it clinically indicated? Then of course, if it is indicated, what are the impacts on one’s life, health, and longevity?
There is a big consideration that needs to be taken into account for many and that is, do I want a family in the future? There are a significant proportion of patients commencing TRT that are less than 40 years of age who are yet to have a family but would like to in the future [1].
In order to understand how TRT can affect fertility, we first need to understand what fertility is and how our male androgen levels can impact it.
Fertility has been defined as:
“…the inability of a male to make a fertile female pregnant, also for a minimum of at least one year of unprotected intercourse.” [2].
So how does male fertility work? In order to understand this, we need to do a little revision on the Hypothalamic Pituitary Gonadal (HPG) Axis. So as not to re-invent the wheel, the below is an excerpt from the article written by Joseph Hearnshaw, The Men’s Health Clinic (TMHC) resident medical researcher.
As a recap at the most basic level, the following happens (I’ve highlighted the most important bits – feel free to read these parts only):
- The hypothalamus, stimulated by the medial preoptic nucleus, secretes Gonadotropin-releasing hormone (GnRH) into the hypophysial portal bloodstream at the medial eminence, which is carried to the pituitary gland. This is pulsatile in nature, i.e., it happens at regular intervals.
- GnRH stimulates the synthesis and secretion of follicle-stimulating hormone (FSH) and luteinising hormone (LH) in the pituitary gland. A low frequency GnRH pulse ensures FSH release, whereas a high-frequency pulse stimulates LH pulses.
- LH stimulates the Leydig cells of the testes to produce Testosterone. FSH acts synergistically here. Intratesticular oestradiol is also produced, as is inhibin, which negatively feeds back to inhibit to production of FSH in the pituitary gland.
- Both the liver and adipose tissue act as major sites for the conversion of testosterone to oestradiol via the aromatase enzyme.
- Oestradiol and Testosterone both feedback negatively to inhibit the production of GnRH at the Hypothalamus, as well as LH and FSH production and secretion at the pituitary gland.
As the output of T and E2 drop due to inhibition, an increase in GnRh and subsequent LH and FSH can occur, in normal healthy individuals. I’ve created a diagram to represent these steps below:
As is outlined above, the negative feedback mechanism by which our HPG axis operates when functioning in a healthy manner is pivotal to ensuring hormonal balance. When one commences TRT this negative feedback loop is interrupted. Essentially the brain recognises that Testosterone is present in the body and dutifully “turns off” the messaging system that causes the secretion of LH and FSH. This is known as pituitary suppression and is an expected result of being on TRT. So, what does this mean for patients receiving TRT? Quite simply this will lead to issues with fertility. Unless of course Human Chorionic Gonadotropin (HCG) is utilised alongside the Testosterone.
Male Fertility
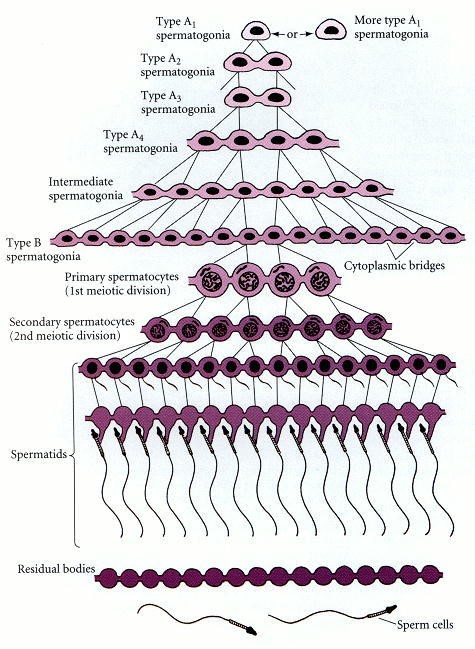
Fertility in males is a complex process. The process of spermatogenesis essentially involves a 10-step process of cell division of both Mitotic and Meiotic nature as can be seen below:
The process of sperm maturation utilises both LH and FSH. The role of FSH is both independent and in unison with LH and its subsequent effect on the Leydig cells and their synthesis of intratesticular testosterone. However, FSH’s role in sperm maturation, proliferation and the delivery of nutrients to germ cells is not a requirement for the process to complete and therefore lead to fertility. This was demonstrated in a rodent model study which showed that rats deficient in FSH but with adequate levels of intratesticular testosterone were able to maintain fertility. Albeit the sperm count of these study participants was decreased relative to the control models [3]. The reason that fertility was preserved in the absence FSH is hypothesised to be related to the presence of intratesticular testosterone allowing for the continued synthesis of Androgen Binding Protein (ABP). The presence of both intratesticular testosterone and LH is what is thought to allow for the continued synthesis of ABP that allows for spermatogenesis to be maintained [4].
The use of HCG alongside testosterone cypionate allows for the mimicking of LH. HCG shares an identical alpha sub-unit with LH. Ultimately this allows it to bind to LH receptors in the testicles and thus stimulate the Leydig cells allowing the production of intratesticular testosterone and thus ABP allowing for the maintenance of a level of fertility. Furthermore, the importance of using a LH analogue such as HCG rather than merely attempting to replace FSH alone to manage fertility issues is supported by a study [5] which looked at the use of Recombinant FSH (rFSH) alongside TRT in the initiation and maintenance of fertility in men with hypogonadism, It concluded that the use of rFSH alongside testosterone replacement therapy without a LH analogue such as HCG yielded no improvement in fertility using baseline azoospermia as a primary outcome measure. Conversely the use of HCG alone demonstrated increases in testosterone levels and spermatogenesis with the addition of rFSH providing further improvements [6]. It therefore stands to reason that the normalisation of male androgen levels by use of small doses of both exogenous testosterone and HCG allows for the preservation of a level of fertility not available to those on monotherapy alone.
Now we cannot discuss the preservation of fertility in the context of TRT without giving mention to the commonly used practice of both Clomiphene Citrate (CC) and HCG Monotherapy [7] [8]. Patients, especially those from a younger demographic who have plans for family in the future but suffer with the typical symptoms of hypogonadism, are often offered treatment with either CC or HCG. In fact, this is the current guidance from the British Society for Sexual Medicine (BSSM) [9].
Let’s look at CC. It falls under the group of medications called Selective Estrogen Receptor Modulators (SERMS). The physiological action of CC, or to give it it’s medical nomenclature “Mechanism of Action” (MOA) is thought to be multifaceted. Essentially its primary action is to selectively bind to estrogen receptors in multiple sites in the body. In the context of TRT in males, the site of primary concern is the hypothalamus. It is here that CC acts as a partial estrogen agonist resulting in estrogenic negative feedback inhibition which, in turn, increases the synthesis of gonadotropins, both LH and FSH and thus increased levels of intratesticular testosterone [10]. Now that we understand the theory behind how CC monotherapy works, what results do we actually see in clinical practice? Well in terms of fertility improvement alone, success rates in improving spermatogenesis do go up. Equally, when looking at serum total testosterone levels, improvements can also be seen. However, in those men that are concurrently suffering with the negative symptoms associated with hypogonadism, we do not tend to see an improvement in reported symptoms. These findings which we, as clinicians, see anecdotally are also reported in the literature [11].
The MOA of HCG has been discussed above. When it comes to HCG monotherapy, similar success rates in the improvement of fertility to CC are seen in terms of quantitative metrics, i.e. improvement in sperm count. In terms of reversal of negative symptoms associated with hypogonadism, anecdotally we see improvements in overall wellbeing and libido but often little in terms of energy and improvement in mental clarity. It stands to reason that one might consider that combining CC and HCG may therefore be effective.
This has been looked at before [12]. Whilst some improvements were seen in the reversal of some negative psychological symptoms. The improvement in sperm count noted in this study was merely superficial as, upon closer inspection, the majority of those sperm had abnormal morphology, i.e. they were physically deformed and therefore not viable.
The use of HCG, CC and indeed other compounds such as Mesterelone (see here for more information), either in isolation or combination therapy, will always lead to dysregulation of the HPG axis. As each individual’s response to interruptions in the normal function of the HPG axis differs, it is difficult to predict the outcomes of such interventions. In our clinical experience at TMHC we often see patients who have been prescribed a “soft approach” to normalisation of very mildly low male androgen levels. The results we see in these cases are nearly always that they end up requiring TRT as a direct result of utilising poor protocols such as either monotherapy of CC/HCG/Mesterolone or any combination thereof.
Those who commence TRT with The Men’s Health Clinic will receive our gold standard protocol of daily micro-dosing of Testosterone Cypionate and HCG via subcutaneous injection. Not only does this allow for a balanced male androgen profile, but will also allow for the preservation of fertility whilst reversing the negative symptoms associated with low male androgen levels. You can read more about our old Standard Therapy here.
References
[1] Handelsman, D., 2013. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Medical Journal of Australia, 199(8), pp.548-551.
[2] Leslie, S., Siref, L., Soon-Sutton, T. and Khan, M., 2021. Male Infertility. [online] Ncbi.nlm.nih.gov. Available at: <https://www.ncbi.nlm.nih.gov/books/NBK562258/> [Accessed 29 December 2021].
[3] Kumar, T., Wang, Y., Lu, N. and Matzuk, M., 1997. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature Genetics, 15(2), pp.201-204.
[4] Oduwole, O., Peltoketo, H. and Huhtaniemi, I., 2018. Role of Follicle-Stimulating Hormone in Spermatogenesis. Frontiers in Endocrinology, 9.
[5] Schaison, G., Young, J., Pholsena, M., Nahoul, K. and Couzinet, B., 1993. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. The Journal of Clinical Endocrinology & Metabolism, 77(6), pp.1545-1549.
[6] Sokol, R., 2009. Endocrinology of Male Infertility: Evaluation and Treatment. Seminars in Reproductive Medicine, 27(02), pp.149-158.
[7] Willets, A., Corbo, J. and Brown, J., 2012. Clomiphene for the Treatment of Male Infertility. Reproductive Sciences, 20(7), pp.739-744.
[8] Lee, J. and Ramasamy, R., 2018. Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Translational Andrology and Urology, 7(S3), pp.S348-S352.
[9] Hackett G, Kirby M, Rees RW, Jones TH, Muneer A, Livingston M, Ossei-Gerning N, David J, Foster J, Kalra PA, Ramachandran S. The British Society for Sexual Medicine Guidelines on Male Adult Testosterone Deficiency, with Statements for Practice. World J Mens Health. 2023 Feb 22. doi: 10.5534/wjmh.221027. Epub ahead of print. PMID: 36876744. Available at: <https://wjmh.org/pdf/10.5534/wjmh.221027> [Accessed 26 April 2023].
[10] Wallach, E. and Adashi, E., 1984. Clomiphene citrate: mechanism(s) and site(s) of action—a hypothesis revisited*. Fertility and Sterility, 42(3), pp.331-344.
[11] Chandrapal, J., Nielson, S., Patel, D., Zhang, C., Presson, A., Brant, W., Myers, J. and Hotaling, J., 2016. Characterising the safety of clomiphene citrate in male patients through prostate-specific antigen, haematocrit, and testosterone levels. BJU International, 118(6), pp.994-1000.
[12] Trinh, T., Hung, N., Hien, L., Tuan, N., Pho, D., Dung, Q., Do, D., Quang, H., Ai, H. and Hung, P., 2021. Evaluating the Combination of Human Chorionic Gonadotropin and Clomiphene Citrate in Treatment of Male Hypogonadotropic Hypogonadism: A Prospective Study. Research and Reports in Urology, Volume 13, pp.357-366.