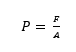TRT & Erythrocytosis – Facts and Recommendations
You may have met doctors afraid of prescribing TRT for fear of raising haematocrit (HCT) too much, but often with a misunderstanding of the underlying cause; an inappropriate TRT regime. In this blog, I’ll be explaining what erythrocytosis is, the different types of erythrocytosis, what HCT is, the risks associated with raised HCT, its causes, and what role TRT plays in all of this. We’ll finalise with how we can manage elevated HCT and ensure we remain physiologically normal.
What is Erythrocytosis?
Erythrocytosis, commonly called polycythaemia, refers to the increase in absolute red blood cell (RBC) mass in your blood. Generally, this will be reflected by an increase in haemoglobin concentration, or haematocrit (the ratio of the volume of RBC to total volume of blood), above what would otherwise be physiologically normal for your age 1.
For RBC mass, the standard generally doesn’t exceed 36 ml/kg in men. For haemoglobin, the reference ranges will vary depending on your genetics, ethnicity, and the altitude you live in or grew up in. But generally, in men, a healthy haemoglobin and haematocrit level will be 16% ± 2mg/dl and 47% ± 6%, respectively. Before discussing the causes of polycythaemia, it’s important to understand that they are different types of polycythaemia. For example, this includes polycythaemia Vera (PV). PV is a sub-type of polycythaemia, and it is an acquired, Philadelphia-chromosome negative, myeloproliferative disorder associated with the overproduction of all blood cell lines (platelets, white blood cells, and RBCs), but most notably RBC 2.
If I lost you at ‘Philadelphia-chromosome’, don’t worry – I’ll explain. Put simply, Philadelphia chromosome, or Ph, is a genetic abnormality in chromosome 22 of leukaemia cancer cells – essentially a mutation within an important chromosome that controls RBC production, where a chromosome is a thread-like structure containing your DNA, all tightly packed up and wrapped around proteins called histones. I’ll spare the complex molecular details, but the bottom line is that the mutation to this important chromosome causes uncontrolled RBC cell division, leading to a slow-growth blood cancer. It generally starts at the age of 60 onwards and can be fatal if not treated 2. So, don’t confused polycythaemia with PV, but stay calm and remember that it’s generally caused by a genetic disease.
Nonetheless, polycythaemia can be fatal via the same underlying mechanism; hyperviscosity of the blood (thick and sticky blood) and it requires treatment. So, what other types of polycythaemia are there?
Polycythaemia Classification & Some Causes
Spurious Polycythaemia
Spurious Polycythaemia is quite different to normal Polycythaemia in that the RBC mass is normal, but a person’s plasma level has decreased 3. Your HCT and haemoglobin concentration will appear high and give a false impression that there’s too many RBCs.
This can be caused by being severely dehydrated, often resulting from isolated fluid loss as seen with diarrhoea and severe vomiting.
Excessive use of diuretics can cause this, as can consumption of cigarettes, excessive alcohol, and excess caffeine. Don’t worry, as long as you hydrate, you’ll be okay 1.
True Polycythaemia
True Polycythaemia is the opposite of spurious; it’s when haematocrit, and/or haemoglobin are elevated. It can be stratified further based upon the serum erythropoietin (EPO) levels present in your blood as follows 1:
Primary Polycythaemia (Low Serum EPO Levels):
- Polycythaemia Vera
- Primary familial and congenital polycythaemia (runs through family, or present since birth; often genetic)
Secondary Polycythaemia (High Serum EPO Levels):
- Caused by high altitude.
- Respiratory disorder related, including Chronic obstructive pulmonary disease (COPD).
- Renal disease related, including renal cancer and renal cysts.
- Elevated carboxyhaemoglobin (common in smokers due to increased carbon monoxide in the blood).
- EPO-secreting tumours.
- Iatrogenic – including use of EPO, supraphysiological use of testosterone, and improper TRT regime.
You’ll notice I mentioned EPO, and it’s by now a well-known hormone popularized by the ‘Lance Armstrong’ Tour de France scandals. EPO is an essential hormone that’s secreted by the kidney in response to cellular hypoxia (decreased oxygen) and it stimulates RBC production. It’s an incredibly important hormone to this topic and your health that we need to discuss further, so we’ll investigate how RBCs are made.
Why We Need RBCs & How They Are Made – A Quick Physiology Lesson
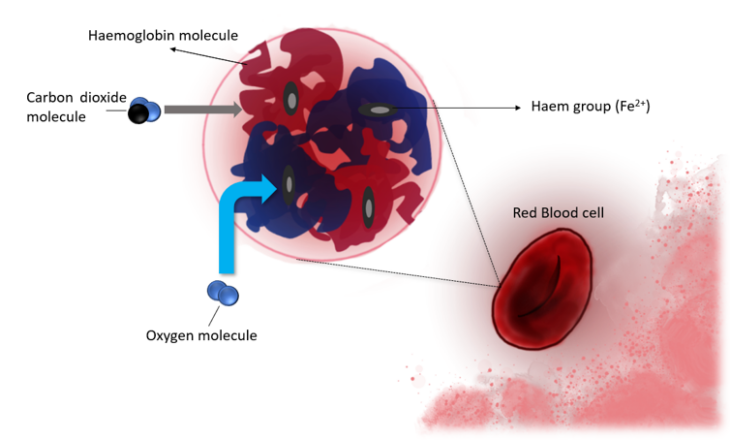
You’ve probably noticed that blood is coloured in some shade of red. This is because the main constitute of our blood is the haemoglobin of RBCs, which is a protein that forms a complex with iron molecules. Haemoglobin is composed of four polypeptides (a chain of amino acids), with each bound to the red pigmented haem. Each haem binds oxygen to the iron ferrous molecule (Fe2+). Each polypeptide changes shape when an oxygen (O2) molecule binds to it, facilitating the binding of another oxygen molecule at another haem site. This makes a ‘complex’ of haem and O2, which is transported from the lungs (when we breath in air) to tissue. Carbon dioxide (CO2) will bind to the polypeptide chains and change the shape of the haemoglobin again to release the O2. The CO2 is then transported to the lungs in the bloodstream, to be breathed out (expired). The exchange of O2 and CO2 depends on the tissue and the concentration of the gases; the lungs have more O2 and less CO2, whereas it’s the opposite in other tissue. RBC facilitate this entire process and are therefore essential to ensure you stay alive.
The structure of haemoglobin and an RBC looks a bit like the below, where CO2 will bind to the polypeptide, and O2 binds to the haem group (click for full size image):
The red and blue messy bits that I’ve crudely drawn would be the polypeptide chains, where the blue blob would be a beta-chain, and the red blob an alpha-chain based polypeptide. Don’t worry about the differences, just know that they’re configured this way to form the 3D shape necessary help transport O2 to tissue and CO2 to the lungs.
How RBCs Are Made
RBCs come from our bone marrow, and the term that describes the production of RBCs is called erythropoiesis. You’ve probably heard of stem cells, and that’s where we start with the process of making RBCs. Stem cells can turn into any cell in the body, if programmed to do so.
In the bone marrow, hematopoietic stem cells (destined to be blood cells) are converted to proerythroblasts, which is then converted to an early erythroblast. During this stage there’s a lot of ‘ribosome synthesis’, where ribosomes are like a factory for protein synthesis. This is important, because this is where haemoglobin is produced and then incorporated into the erythroblast to form a ‘late’ or erythroblast. This late erythroblast then gets converted into a ‘normoblast’, which then loses its nucleus (the centre of a cell with all the important DNA) to form a reticulocyte. It Takes ~3 days for the reticulocyte to enter our bloodstream, where in ~24 hours it’ll form an erythrocyte; an RBC. The erythrocyte will last for around 120 days or less (if damaged), until it’s removed by either the spleen, liver, or bone marrow. For a little perspective, the human body will generate approximately 2 million RBCs every second 4.
This process would look a bit like the below, from the bone marrow to the blood vessel (click for full size image):
Also, it’s worth noting that iron is important to produce these Erythroblasts to be normal. As is folate and vitamin B12, which facilitate the proliferation (rapid reproduction) and differentiation (conversion, ultimately, to an RBC) of Erythroblasts. This is best gained from your diet; green vegetables and meats. But get a blood test to see where you stand, especially if you’re vegan or vegetarian as you may struggle more to get iron and vitamin B12 in your diet 5.
Great! So What Regulates This Whole Process & What Does EPO Have To Do With It?
As mentioned earlier, polycythaemia can be related to your serum EPO levels, and EPO can stimulate RBC production. This is its primary function – it helps regulate oxygen delivery to peripheral tissues and is stimulated by hypoxia (lack of Oxygen). A few scenarios can cause this, including smoking (displacing oxygen with carbon monoxide from smoke) and living in high altitudes where there’s less Oxygen.
An erythropoietin receptor (EPO-R) exists on the outside of, which EPO can bind to, form a ‘homodimer’, and activate a signalling pathway; the JAK2/STAT5 pathway. This leads to the production of Megakaryocyte-erythroid progenitor cells (MEP) and their survival. Remember stem cells? MEPs are committed self-renewing stem cells that give rise to RBCs – more MEPs means more RBCs produced in the bone marrow 6,7 ! Remember we mentioned PV earlier, too? Well it is often also caused by a mutation to the JAK2 gene 8 – this leads to the pathway being chronically activated, making too many MEPs, meaning too many RBCs, as demonstrated nicely by the image below 9 (click for full size image):
The Risk of Elevated HCT and/or Haemoglobin
With an increase in RBCs and subsequent HCT, we will ultimately see an increase in arterial blood pressure. This is due to an increase in blood viscosity and the reversal of hypoxic vasodilation 10. Hypoxia causes blood vessels to relax and increase in diameter, which will lower blood pressure due to having an increased area, where pressure is defined as force divided by the area:
Furthermore, high haemoglobin concentration, or HCT, is a known risk factor or major atherosclerotic cardiovascular events (i.e. ischemic strokes, acute myocardial infection or heart attack, and congestive heart failure) 11. This will be due to elevated blood pressure, cardiac load, and friction with the blood vessel wall, leading to damage and build-up of atherosclerotic plaque as a result in men 12.
So, you may think that keeping our haemoglobin low would be better, and some studies suggest this such as in one investigating low versus normal Haemoglobin in a Chinese population 13. Conversely, some studies show that low haemoglobin levels are associated with an increased risk of chronic kidney disease in a Korean sample of half a million healthy adults 14. Too low and we risk inadequate haemoglobin for oxygenation of tissue, and we can cause iron deficiency. Too high and we will increase blood viscosity and cardiac load, which can lead to cardiac diseases. But again, we need to consider this on an individual basis; those who have had ancestral roots in mountainous regions will likely naturally have a higher HCT/Haemoglobin. We should strive to remain within our reference range for HCT/Haemoglobin and check our RDW, ensuring it doesn’t fall below 10.2% which indicates potential microcytic anaemia. We should also pay attention to our MCH, if it’s low then we risk microcytic anaemia; in this situation you should get a blood test for vitamin B12, iron, and folate.
Ironically, athletes will see an immediate decrease in haematocrit post training due to increase plasma volume, which leads to ‘sports anaemia’ which isn’t detrimental to performance. True anaemia is however common among athletes due to iron deficiency, especially in female athletes. Though, in the long term they have will also have a greater HCT and RBC mass compared to sedentary individuals, as they require more oxygen; a by-product of training 15,16. Still, excess HCT/Haemoglobin will cause issues even in athletes; it’s not healthy in the long run.
How is TRT Involved & How Can We Manage TRT Related Polycythaemia?
Erythrocytosis is the most commonly reported adverse event that occurs in testosterone trials, often a result of the TRT protocol 17. So how does testosterone stimulate erythropoiesis?
It’s suggested that it does this in part via stimulating EPO production. Testosterone can also act directly on the bone marrow and increase the number of EPO-responsive cells 18,19. It’s also suggested that the concurrent suppression of hepcidin via Testosterone, and elevated EPO, can lead to increased HCT 20; Testosterone lowers hepcidin, a regulator of iron bioavailability. Though, whether testosterone does this via stimulating EPO production is debated among studies and no clear consensus has been reached. There’s even some suggestion that testosterone may mediate RBC increase via synthesis of insulin-growth like factor 1 (IGF-1) 21,22.
Recall I mentioned that hypoxia is a cause of polycythaemia. Chronic obstructive pulmonary disease (COPD) can also cause this and explains the elevated haematocrit we seen in men with COPD. TRT can improve COPD via improvements in body composition that occurs over months often sees a reduction in COPD over time, provided adequate weight is lost and the patient exercises 23. The most common cause of COPD is Tobacco smoking, high air pollution, exposure to dust and fumes, overweightness and obesity, and genetics – where an Alpha1-antitrypsin deficiency is a known genetic cause of COPD, occurring in ~1-2% of all COPD cases 24. Other genes are also contributory. Losing weight and cigarettes are the best thing you can do to prevent COPD and subsequent COPD induced polycythaemia.
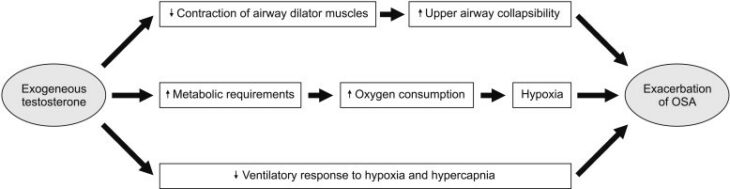
Unfortunately, Obstructive Sleep Apnoea (OSA) may occur with TRT and is characterized by intermittent hypoxia and poor sleep 25. This can raise HCT or even cause secondary polycythaemia, but it’s generally a side effect in certain patients and it’s suggested to occur via central and peripheral mechanisms (figure 1 – click for full size image).
Figure 1 – Plausible mechanisms by which TRT may worsen OSA 25
A supraphysiological dose of Testosterone would worsen this further, so a solution is finding the correct dosage and reducing other risk factors known to contribute to OSA such as weight, or via treatment with a CPAP device. OSA often impairs natural testosterone levels due to disturbed sleep and hypoxia, which may well result in weight gain and subsequent worsening of OSA.
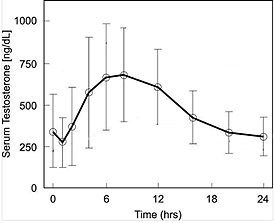
We’ll be coming back to the topic of micro-dosing, as discussed in the previous blog. But, let me first explain the natural diurnal rhythm of Testosterone that we normally see in healthy men not on TRT. Naturally, your testosterone will be at its highest upon waking up, and subsequently decrease as the day progresses through a 24-hour period, looking a bit like the below (figure 2 – click for full size image):
Figure 2 – Diurnal pattern of testosterone over a 24-hour period in a healthy male
This is also concurrent with the fact that we go through catabolic and anabolic states throughout the day and night; we don’t always remain one or the other, naturally. Cortisol, growth hormone, and Thyroid hormones also follow a diurnal pattern. As we get older, the diurnal variation lessens. This generally causes a rise in SHBG and lower Testosterone, generally due to cells being exhausted and perhaps from an evolutionary point of view, normally being done with procreation.
One issue with bolus dosing, i.e. taking Testosterone Cypionate once or twice a week, is that we acutely increase Testosterone in most cases, with a much higher increase versus micro dosing, due to a higher dose being used.
It’s suspected that with a sudden raise in free Testosterone, the kidneys will respond by increasing EPO dramatically, and we’ll see an increase in EPO-responsive cells in the bone marrow. Likewise, hepcidin would decrease. Conversely, a more stable testosterone administration routine would prevent an acute and excessive rise, and lead to more stable and manageable haematocrit. Ergo, it’s preferable to use micro-dosing. Sadly, no studies exist for this and we can only rely on our own experience and data so far. But a study demonstrating this is well within the possibilities of being published in the future.
So other than micro-dosing, what else can you do? Well, if you have polycythaemia then you can get therapeutic venesection (removing blood) to treat it. If you’re just generally trying to control HCT and have no known factors that would prevent you from donating blood, then donate blood; being on medically prescribed TRT will not prevent you from being able to donate blood. But first read on whether you’re eligible to donate blood at https://www.blood.co.uk for any other factors that may make you non-eligible. You’d be doing your health and the health of another person who needs that blood a great service!
References
- Babiker. AAPHM. Polycythemia. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK526081/. Published 2019. Accessed January 22, 2020.
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14-22. doi:10.1038/sj.leu.2404955
- Diagnosing or Ruling Out Polycythemia Vera in Patients With Erythrocytosis – Hematology & Oncology. https://www.hematologyandoncology.net/archives/january-2019/diagnosing-or-ruling-out-polycythemia-vera-in-patients-with-erythrocytosis/. Accessed January 23, 2020.
- Zivot A, Lipton JM, Narla A, Blanc L. Erythropoiesis: Insights into pathophysiology and treatments in 2017. Mol Med. 2018;24(1). doi:10.1186/s10020-018-0011-z
- Koury MJ, Ponka P. NEW INSIGHTS INTO ERYTHROPOIESIS: The Roles of Folate, Vitamin B 12 , and Iron . Annu Rev Nutr. 2004;24(1):105-131. doi:10.1146/annurev.nutr.24.012003.132306
- Mulcahy L. The erythropoietin receptor. Semin Oncol. 2001;28(2 Suppl 8):19-23. doi:10.1016/s0093-7754(01)90208-8
- Erythroid Progenitor Cells Development in the Hematopoietic Bone Marrow – LifeMap Discovery. https://discovery.lifemapsc.com/in-vivo-development/blood/hematopoietic-bone-marrow/erythroid-progenitor-cells. Accessed January 25, 2020.
- Raedler LA. Diagnosis and Management of Polycythemia Vera: Proceedings from a Multidisciplinary Roundtable. Am Heal drug benefits. 2014;7(7 Suppl 3):S36-47. http://www.ncbi.nlm.nih.gov/pubmed/26568781. Accessed January 25, 2020.
- Disease Overview. https://www.jakavi.com/en/polycythemia-vera/about-pv/disease-overview/. Accessed January 25, 2020.
- Raine AE, Roger SD. Effects of erythropoietin on blood pressure. Am J Kidney Dis. 1991;18(4 Suppl 1):76-83. http://www.ncbi.nlm.nih.gov/pubmed/1928084. Accessed January 25, 2020.
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. High blood hemoglobin concentration as risk factor of major atherosclerotic cardiovascular events in 114,159 healthy men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Ann Med. 2012;44(5):476-486. doi:10.3109/07853890.2011.573804
- Irace C, Ciamei M, Crivaro A, et al. Hematocrit is associated with carotid atherosclerosis in men but not in women. Coron Artery Dis. 2003;14(4):279-284. doi:10.1097/01.mca.0000071769.74379.49
- Ren L, Gu B, Du Y, et al. Hemoglobin in normal range, the lower the better?-Evidence from a study from Chinese community-dwelling participants. J Thorac Dis. 2014;6(5):477-482. doi:10.3978/j.issn.2072-1439.2014.02.12
- Yi SW, Moon SJ, Yi JJ. Low-normal hemoglobin levels and anemia are associated with increased risk of end-stage renal disease in general populations: A prospective cohort study. PLoS One. 2019;14(4). doi:10.1371/journal.pone.0215920
- Eichner ER. Sports anemia, iron supplements, and blood doping. Med Sci Sports Exerc. 1992;24(9):S315-S318. doi:10.1249/00005768-199209001-00002
- Mairbäurl H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4 NOV. doi:10.3389/fphys.2013.00332
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. Journals Gerontol – Ser A Biol Sci Med Sci. 2005;60(11):1451-1457. doi:10.1093/gerona/60.11.1451
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93(3):914-919. doi:10.1210/jc.2007-1692
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. Journals Gerontol – Ser A Biol Sci Med Sci. 2014;69(6):725-735. doi:10.1093/gerona/glt154
- Bachman E, Feng R, Travison T, et al. Testosterone suppresses hepcidin in men: A potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95(10):4743-4747. doi:10.1210/jc.2010-0864
- Mechanism of Action of Androgens on Erythropoiesis – A Review. https://www.researchgate.net/publication/311086229_Mechanism_of_Action_of_Androgens_on_Erythropoiesis_-_A_Review. Accessed January 25, 2020.
- Singh VK, Saini A, Chandra R. Role of Erythropoietin and Other Growth Factors in Ex Vivo Erythropoiesis . Adv Regen Med. 2014;2014:1-8. doi:10.1155/2014/426520
- Svartberg J, Aaseboø U, Hjalmarsen A, Sundsfjord J, Jorde R. Testosterone treatment improves body composition and sexual function in men with COPD, in a 6-month randomized controlled trial. Respir Med. 2004;98(9):906-913. doi:10.1016/j.rmed.2004.02.015
- Vijayan VK. Chronic obstructive pulmonary disease. Indian J Med Res. 2013;137(2):251-269. http://www.ncbi.nlm.nih.gov/pubmed/23563369. Accessed January 25, 2020.
- Kim S-D, Cho K-S. Obstructive Sleep Apnea and Testosterone Deficiency. World J Mens Health. 2019;37(1):12. doi:10.5534/wjmh.180017